Textured Implants Recalled By The FDA, Linked To A Rare Kind Of Cancer9 min read


Phillip Chang, MD
Board Certified By The American Board of Plastic Surgeons.
Voted Top Plastic Surgeon in Loudoun, Virginia
Offices in Leesburg, Virginia.



An article in The New York Times was published last week talking about a specific breast implant that is being recalled due to a link to a rare kind of cancer. Textured breast implants made by Allergan are being recalled in the US by the Food and Drug Administration (FDA). The link to cancer is not ‘new’ news, in fact this has been a talking point for many months. The implants are already banned in Europe where textured implants were the more popular form of breast implant. In the US, only 5% of breast implants used are textured with smooth implants being the popular choice of most patients and surgeons.
The rare cancer is not actually breast cancer: it develops in the tissue surrounding the implant. It’s name is Anaplastic Large-Cell Lymphoma (ALCL) and has occurred with implants for both cosmetic and reconstruction surgeries. In most cases removal of the implant and cancerous tissue cures the patient. The key is early detection. If you have textured implants and you are experiencing swelling and fluid retention, seek professional help.
I have been following this subject for months and can assure my patients that I DO NOT USE textured implants for breast augmentation surgeries. I want to educate my readers and clients on the differences between smooth and textured and give them all the information they need to make educated decisions on their implants or future implants.
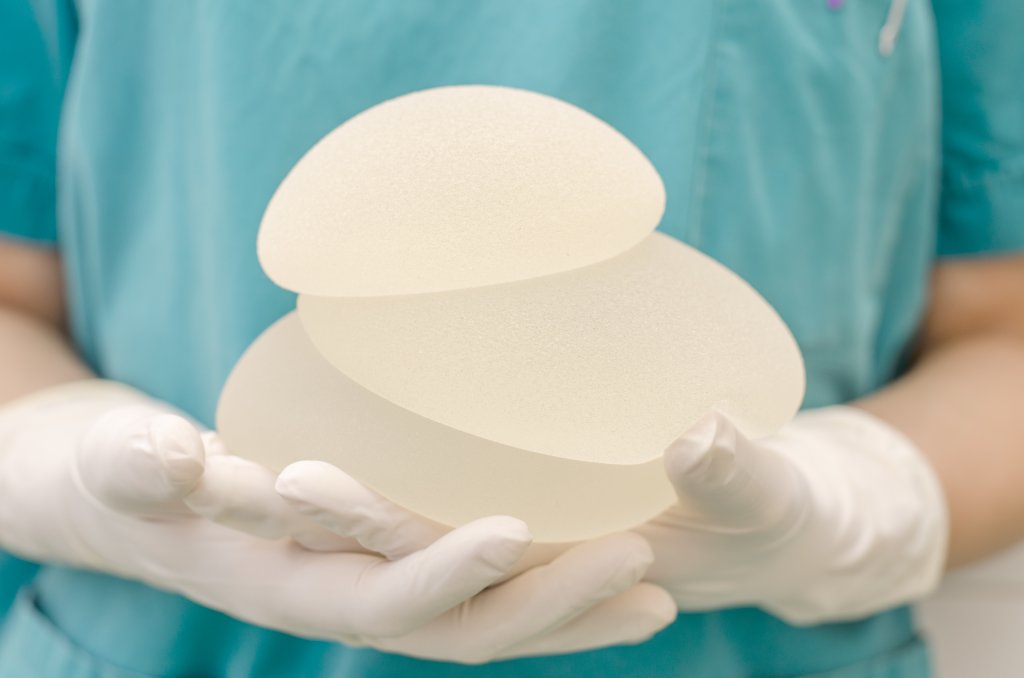
Popularity: Smooth Versus Textured Implants In The United States
Boob jobs (or breast augmentation to give it the medical term) is one of the most popular surgeries in the US. About 300,000 women a year get breast implants in the United States. That’s a 4% increase on the year before. Most of those ladies are getting it done for cosmetic reasons with a smaller minority getting breast augmentation surgery for reconstruction purposes.
There are a number of options available to women and your first consultation with your plastic surgeon will be spent going over those choices. Options like size, type of implant – saline or silicone, round or shaped implants and finally textured or smooth.Your surgeon will cover the pros and cons of each option and show you examples of all so you can get a feel of what is being into your body.
In the United States, the vast majority of breast implants placed are smooth, non-textured breast implants. Europe and the rest of the world traditionally used textured breast implants over smooth breast implants. Although with the ban of Allergan in Europe this has changed in the last year.
The safety of breast implants has been in the news before with reports that breast implants may cause breast cancer. Note that the risk for cancer in the breast appears to be mainly related to TEXTURED breast implants. Please note that the cancer in the news is not breast cancer, it is a form of lymphoma. Also note that this cancer ALCL is extremely rare.
Note that the risk for cancer in the breast appears to be mainly related to TEXTURED breast implants. Please note that the cancer in the news is not breast cancer, it is a form of lymphoma. Also note that this cancer ALCL is extremely rare.
Breast Augmentations Don't Need Textured Implants
The goal of a textured implant was to increase surface area and tension, which supposedly decreased the risk of developing a thicker capsule around the implant. They were also designed to prevent moving inside the breast, the rough texture having a better purchase on the breast tissue. This was particularly relevant when implanting tear-drop shaped implants. Any rotation would cause the breast to distort.
Personally, I prefer not to use tear-drop shaped implants. As they are flatter at the top, many women choose not to have them. After childbirth and breast feeding, women’s breasts become flatter at the top, and they want to regain the fullness they had before. A tear-drop implant will not provide that fullness.
Breast Cancer and Saline or Silicone Implants
Saline and silicone implants do not increase the risks of getting breast cancer according to Susan Komen, a prominent breast cancer advocate. The organization, regularly reviews data surrounding breast cancer and breast implants and publishes their findings on their website. Many of the studies showed a lower risk of breast cancer among women with implants.
What Does the FDA Say About Breast Cancer And Breast Implants?
The FDA announced the recall on July 24, 2019 stating their decision was based on the increasing number of cases and deaths associated to the Allergan implants. Of the 573 cases reported, 481 were attributed to Allergan implants and 12 deaths were of patients with textured implants from Allergan.
“Although the overall incidence of BIA-ALCL appears to be relatively low, once the evidence indicated that a specific manufacturer’s product appeared to be directly linked to significant patient harm, including death, the FDA took action to alert the firm to new evidence indicating a recall is warranted to protect women’s health,” said FDA Principal Deputy Commissioner Amy Abernethy, M.D., Ph.D.
They also stated that the majority of women who develop the implant-related cancer have TEXTURED implants, but there are some cases in which cancer patients have implants with smooth surfaces. In many of the lymphoma cases, the agency doesn’t know whether the implants were smooth or textured.
The majority of women who develop the implant-related cancer have TEXTURED implants.
FDA Recommendations To Health Care Providers
- If patients show evidence of pain, lumps, swelling, breast assymetry, or the development of a fluid collection (seroma) around the breast implant they should book a consultation with a qualified plastic surgeon to talk over their options.
- If fluid collection has occured, a sample should be taken and sent away for pathology testing to rule out ALCL.
- Reports of ALCL should be sent through to the FDA so they can have updated data to make further decisions and give accurate guidance.
- Be sure to send case reports to the Plastic Surgery Foundation’s PROFILE registry.
FDA recommendations to patients
- Before your breast augmentation, learn about the varieties of breast implants available.
- If you have textured implants and have NO symptoms, they do not recommend removal.
- Keep in mind ALCL appears to develop more frequently in individuals with textured implants than in people with smooth-surfaced implants. The cost and benefits of textured vs smooth implants is a great topic to bring up with your health care provider!
- Swelling and pain are normal effects of breast augmentation surgery. But call your doctor right away if you notice anything unusual post-recovery like swelling or pain in the implant vicinity. You should also call your doctor if your breasts look or feel different suddenly.
What Does Allergan Say?
Allergan announced after the recall that they would be halting sales of the following textured Biocell implants worldwide:
- Natrelle Saline-Filled breast implants.
- Natrelle Silicone-Filled breast implants.
- Natrelle Inspira Silicone-Filled breast implants.
- Natrelle 410 Highly Cohesive Anatomically Shaped Silicone-Filled breast implants.
The recall also includes tissue expanders used by patients prior to breast augmentation or reconstruction, including:
- Natrelle 133 Plus Tissue Expander.
- Natrelle 133 Tissue Expander with Suture Tabs.
Allergan stated they have added to their warranty the ability to receive $ 1,000 towards getting the test for ALCL. If the test is positive, then Allergan will contribute $ 7,500 toward the cost of removal and possible replacement of the breast implant. The replacement would NOT be textured. If patients wish to remove their implants regardless of whether they have ALCL, they would need to do so at their own expense as the FDA is not recommending their removal.
FAQs About Breast Implants and Breast Cancer
No, the only reported cases of breast cancer post-surgery is when textured breast implants are used. No recent studies have connected smooth implants and breast cancer.
Not exactly. It is a technically a cancer in the breast but not really a breast cancer . The distinction is that the cells that form the cancer are not breast cells. ALCL is a type of non-Hodgkins lymphoma; it is a cancer of the immune system.
I would argue that in most cases textured implants are unnecessary to achieving your desired aesthetic. Textured implants are largely used for anatomic or tear-drop shaped implants to prevent them from rotating in the breast pocket. But, tear-drop shaped implants are the wrong look for most women.
No, according to Susan Koman. In fact, the organization found that many studies show a LOWER risk of breast cancer among women with implants.
Absolutely contact your health care provider. Some swelling and pain is to be expected after surgery. If you feel any lumps or bumps that are not normal to your breast tissue however you may require some imaging just to be on the safe side. Follow up with your breast surgeon and your health care providers.
Educate yourself and contact us. We would be happy to help you weigh the pros and cons of all the different breast implant options. We want to make these decisions as easy for you as possible!
INVITATION
Do you find this content interesting and want more? Join our newsletter!
For those wondering whether breast augmentation might be the best cosmetic solution for you, we invite you to simply come in for a complimentary consultation with Dr. Chang or one of the cosmetic laser and injection nurses to explore whether you would make a good candidate. To find out more whether Aesthetica can help you, contact us online or at 703-729-5553 to arrange an appointment. Dr. Phillip Chang is a board-certified plastic surgeon in Northern Virginia near Leesburg, Virginia and an expert in a wide variety of cosmetic treatments.
What You Get
Innovations in cosmetic surgery, coupons and specials and invitations to our free seminars
Sign Up For Our Newsletter
More Articles For You

What’s a Unit of BOTOX Anyway? | Aesthetica GoToBeauty
Have you ever heard about BOTOX and wondered what it’s all about? It’s like the
When Do Boobs Stop Growing? Finding the Perfect Time for Breast Surgery
Ever wondered when your boobs finally decide to take a break from growing? Or you’re
Are Silicone Injections in Buttocks Safe?
In today’s world, where the aesthetic appeal of one’s body can often feel as though

Does a Mommy Makeover Include BBL?
Many women look forward to the blessings of motherhood. Having a child is a very
